Easy 3 Credit Courses Uf Nature of Running
Ultrafiltration (UF) is a variety of membrane filtration in which forces like pressure level or concentration gradients atomic number 82 to a separation through a semipermeable membrane. Suspended solids and solutes of loftier molecular weight are retained in the so-called retentate, while water and low molecular weight solutes pass through the membrane in the permeate (filtrate). This separation process is used in industry and research for purifying and concentrating macromolecular (tenthree–106 Da) solutions, peculiarly protein solutions.
Ultrafiltration is not fundamentally dissimilar from microfiltration. Both of these split based on size exclusion or particle capture. Information technology is fundamentally different from membrane gas separation, which dissever based on different amounts of absorption and unlike rates of improvidence. Ultrafiltration membranes are divers by the molecular weight cut-off (MWCO) of the membrane used. Ultrafiltration is applied in cross-flow or expressionless-end style.
Applications [edit]
Industries such as chemic and pharmaceutical manufacturing, food and beverage processing, and waste material water treatment, utilise ultrafiltration in order to recycle flow or add value to later products. Blood dialysis also utilizes ultrafiltration.
Drinking water [edit]

Drinking water treatment 300 mthree/h using ultrafiltration in Grundmühle waterworks (Deutschland)
Ultrafiltration tin can be used for the removal of particulates and macromolecules from raw h2o to produce potable water. It has been used to either replace existing secondary (coagulation, flocculation, sedimentation) and tertiary filtration (sand filtration and chlorination) systems employed in water handling plants or equally standalone systems in isolated regions with growing populations.[one] When treating water with high suspended solids, UF is often integrated into the procedure, utilising primary (screening, flotation, filtration) and some secondary treatments as pre-handling stages.[2] UF processes are currently preferred over traditional handling methods for the following reasons:
-
- No chemicals required (aside from cleaning)
- Constant production quality regardless of feed quality
- Compact plant size
- Capable of exceeding regulatory standards of water quality, achieving 90–100% pathogen removal[3]
UF processes are currently express by the high cost incurred due to membrane fouling and replacement.[4] Additional pretreatment of feed water is required to prevent excessive damage to the membrane units.
In many cases UF is used for pre filtration in reverse osmosis (RO) plants to protect the RO membranes.
Protein concentration [edit]
UF is used extensively in the dairy manufacture;[5] particularly in the processing of cheese whey to obtain whey protein concentrate (WPC) and lactose-rich permeate.[6] [7] In a single phase, a UF process is able to concentrate the whey 10–thirty times the feed.[eight]
The original alternative to membrane filtration of whey was using steam heating followed by pulsate drying or spray drying. The product of these methods had express applications due to its granulated texture and insolubility. Existing methods likewise had inconsistent production limerick, high capital and operating costs and due to the excessive heat used in drying would oft denature some of the proteins.[6]
Compared to traditional methods, UF processes used for this application:[half dozen] [8]
-
- Are more energy efficient
- Take consistent product quality, 35–80% protein product depending on operating atmospheric condition
- Do non denature proteins as they utilise moderate operating conditions
The potential for fouling is widely discussed, being identified equally a significant contributor to decline in productivity.[vi] [7] [eight] Cheese whey contains loftier concentrations of calcium phosphate which tin can potentially lead to calibration deposits on the membrane surface. Every bit a result, substantial pretreatment must exist implemented to balance pH and temperature of the feed to maintain solubility of calcium salts.[8]

Other applications [edit]
-
- Filtration of effluent from paper pulp factory
- Cheese manufacture, meet ultrafiltered milk
- Removal of some bacteria from milk
- Process and waste h2o treatment
- Enzyme recovery
- Fruit juice concentration and clarification
- Dialysis and other blood treatments
- Desalting and solvent-commutation of proteins (via diafiltration)
- Laboratory grade manufacturing
- Radiocarbon dating of bone collagen
Principles [edit]
The basic operating principle of ultrafiltration uses a pressure induced separation of solutes from a solvent through a semi permeable membrane. The relationship between the applied pressure on the solution to be separated and the flux through the membrane is most commonly described by the Darcy equation:
- ,
where J is the flux (period rate per membrane area), TMP is the transmembrane pressure (force per unit area divergence between feed and permeate stream), μ is solvent viscosity and R t is the total resistance (sum of membrane and fouling resistance).
Membrane fouling [edit]
Concentration polarization [edit]
When filtration occurs the local concentration of rejected material at the membrane surface increases and tin can become saturated. In UF, increased ion concentration tin develop an osmotic pressure on the feed side of the membrane. This reduces the effective TMP of the arrangement, therefore reducing permeation rate. The increase in full-bodied layer at the membrane wall decreases the permeate flux, due to increase in resistance which reduces the driving force for solvent to send through membrane surface. CP affects virtually all the available membrane separation processes. In RO, the solutes retained at the membrane layer results in higher osmotic pressure in comparison to the majority stream concentration. So the higher pressures are required to overcome this osmotic pressure. Concentration polarisation plays a dominant office in ultrafiltration as compared to microfiltration because of the small pore size membrane.[9] Concentration polarization differs from fouling as it has no lasting effects on the membrane itself and tin exist reversed by relieving the TMP. It does all the same accept a significant issue on many types of fouling.[10]
Types of fouling [edit]
Particulate deposition [edit]
The following models describe the mechanisms of particulate deposition on the membrane surface and in the pores:
-
- Standard blocking: macromolecules are uniformly deposited on pore walls
- Consummate blocking: membrane pore is completely sealed by a macromolecule
- Cake formation: accumulated particles or macromolecules class a fouling layer on the membrane surface, in UF this is likewise known every bit a gel layer
- Intermediate blocking: when macromolecules deposit into pores or onto already blocked pores, contributing to block formation [11]
Scaling [edit]
As a result of concentration polarization at the membrane surface, increased ion concentrations may exceed solubility thresholds and precipitate on the membrane surface. These inorganic table salt deposits tin cake pores causing flux decline, membrane degradation and loss of product. The formation of scale is highly dependent on factors affecting both solubility and concentration polarization including pH, temperature, flow velocity and permeation charge per unit.[12]
Biofouling [edit]
Microorganisms will adhere to the membrane surface forming a gel layer – known as biofilm.[13] The film increases the resistance to flow, acting as an additional barrier to permeation. In spiral-wound modules, blockages formed by biofilm can lead to uneven flow distribution and thus increment the furnishings of concentration polarization.[xiv]
Membrane arrangements [edit]

Depending on the shape and material of the membrane, unlike modules can be used for ultrafiltration process.[fifteen] Commercially bachelor designs in ultrafiltration modules vary co-ordinate to the required hydrodynamic and economic constraints likewise as the mechanical stability of the organization under particular operating pressures.[16] The principal modules used in industry include:
Tubular modules [edit]
The tubular module design uses polymeric membranes bandage on the within of plastic or porous paper components with diameters typically in the range of 5–25 mm with lengths from 0.vi–6.4 m.[6] Multiple tubes are housed in a PVC or steel trounce. The feed of the module is passed through the tubes, accommodating radial transfer of permeate to the crush side. This design allows for easy cleaning notwithstanding the main drawback is its depression permeability, high book hold-up within the membrane and low packing density.[half-dozen] [16]
Hollow fibre [edit]

Self-supporting hollow fibre module
This design is conceptually similar to the tubular module with a shell and tube organization. A single module can consist of 50 to thousands of hollow fibres and therefore are self-supporting unlike the tubular design. The diameter of each fibre ranges from 0.2–3 mm with the feed flowing in the tube and the production permeate collected radially on the outside. The advantage of having self-supporting membranes as is the ease at which it can exist cleaned due to its power to exist backflushed. Replacement costs however are high, as one faulty fibre will require the whole bundle to be replaced. Considering the tubes are of small diameter, using this design too makes the arrangement prone to blockage.[8]
Screw-wound modules [edit]

Spiral-wound membrane module
Are composed of a combination of flat membrane sheets separated by a thin meshed spacer textile which serves as a porous plastic screen support. These sheets are rolled around a central perforated tube and fitted into a tubular steel pressure vessel casing. The feed solution passes over the membrane surface and the permeate spirals into the central drove tube. Spiral-wound modules are a compact and cheap alternative in ultrafiltration pattern, offer a high volumetric throughput and can likewise be easily cleaned.[sixteen] Nevertheless it is express by the thin channels where feed solutions with suspended solids can result in partial blockage of the membrane pores.[8]
Plate and frame [edit]
This uses a membrane placed on a flat plate separated by a mesh like material. The feed is passed through the system from which permeate is separated and collected from the edge of the plate. Channel length tin can range from 10–60 cm and channel heights from 0.5–1.0 mm.[8] This module provides low book hold-up, relatively easy replacement of the membrane and the ability to feed viscous solutions because of the low channel height, unique to this particular pattern.[16]
Procedure characteristics [edit]
The procedure characteristics of a UF organization are highly dependent on the type of membrane used and its application. Manufacturers' specifications of the membrane tend to limit the process to the following typical specifications:[17] [18] [xix] [20]
| Hollow Fibre | Spiral-wound | Ceramic Tubular | Plate and Frame | |
|---|---|---|---|---|
| pH | 2–13 | 2–11 | iii–7 | |
| Feed Pressure (psi) | nine–xv | <30–120 | 60–100 | |
| Backwash Pressure (psi) | nine–15 | 20–forty | 10–thirty | |
| Temperature (°C) | v–30 | 5–45 | 5–400 | |
| Total Dissolved Solids (mg/L) | <1000 | <600 | <500 | |
| Full Suspended Solids (mg/50) | <500 | <450 | <300 | |
| Turbidity (NTU) | <15 | <ane | <x | |
| Iron (mg/L) | <5 | <five | <5 | |
| Oils and Greases (mg/L) | <0.1 | <0.one | <0.1 | |
| Solvents, phenols (mg/Fifty) | <0.ane | <0.ane | <0.i |
Procedure pattern considerations [edit]
When designing a new membrane separation facility or considering its integration into an existing found, there are many factors which must be considered. For near applications a heuristic arroyo tin can be practical to determine many of these characteristics to simplify the design process. Some design areas include:
Pre-treatment [edit]
Treatment of feed prior to the membrane is essential to foreclose damage to the membrane and minimize the effects of fouling which greatly reduce the efficiency of the separation. Types of pre-treatment are oft dependent on the type of feed and its quality. For example, in wastewater treatment, household waste and other particulates are screened. Other types of pre-treatment common to many UF processes include pH balancing and coagulation.[21] [22] Appropriate sequencing of each pre-treatment phase is crucial in preventing harm to subsequent stages. Pre-handling can even exist employed simply using dosing points.
Membrane specifications [edit]
Material [edit]
About UF membranes use polymer materials (polysulfone, polypropylene, cellulose acetate, polylactic acid) however ceramic membranes are used for high temperature applications.
Pore size [edit]
A general rule for choice of pore size in a UF system is to use a membrane with a pore size one tenth that of the particle size to be separated. This limits the number of smaller particles entering the pores and adsorbing to the pore surface. Instead they block the entrance to the pores assuasive unproblematic adjustments of cross-flow velocity to dislodge them.[eight]
Operation strategy [edit]

Schematic of cantankerous menstruum performance.
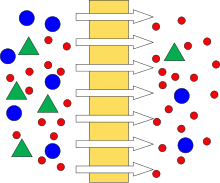
Schematic of dead-end functioning
Flowtype [edit]
UF systems can either operate with cross-period or dead-end menses. In dead-end filtration the flow of the feed solution is perpendicular to the membrane surface. On the other hand, in cross menstruation systems the menses passes parallel to the membrane surface.[23] Expressionless-end configurations are more than suited to batch processes with low suspended solids every bit solids accumulate at the membrane surface therefore requiring frequent backflushes and cleaning to maintain loftier flux. Cross-menstruum configurations are preferred in continuous operations every bit solids are continuously flushed from the membrane surface resulting in a thinner cake layer and lower resistance to permeation.
Flow velocity [edit]
Flow velocity is especially critical for hard water or liquids containing suspensions in preventing excessive fouling. College cross-catamenia velocities tin be used to enhance the sweeping effect across the membrane surface therefore preventing deposition of macromolecules and colloidal material and reducing the furnishings of concentration polarization. Expensive pumps are however required to reach these conditions.
Flow temperature [edit]
To avert excessive damage to the membrane, it is recommended to operate a plant at the temperature specified by the membrane manufacturer. In some instances yet temperatures beyond the recommended region are required to minimise the effects of fouling.[22] Economic assay of the procedure is required to observe a compromise between the increased cost of membrane replacement and productivity of the separation.
Pressure level [edit]
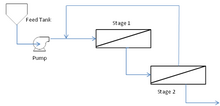
Typical 2 stage membrane process with recycle stream
Force per unit area drops over multi-phase separation can result in a desperate turn down in flux functioning in the latter stages of the process. This can be improved using booster pumps to increase the TMP in the terminal stages. This volition incur a greater majuscule and free energy cost which will be start by the improved productivity of the process.[22] With a multi-stage functioning, retentate streams from each stage are recycled through the previous phase to improve their separation efficiency.
Multi-phase, multi-module [edit]
Multiple stages in series can exist applied to achieve higher purity permeate streams. Due to the modular nature of membrane processes, multiple modules can be arranged in parallel to treat greater volumes.[24]
Post-treatment [edit]
Mail service-treatment of the product streams is dependent on the composition of the permeate and retentate and its end-use or government regulation. In cases such equally milk separation both streams (milk and whey) can be collected and made into useful products. Boosted drying of the retentate volition produce whey pulverization. In the paper factory manufacture, the retentate (not-biodegradable organic material) is incinerated to recover energy and permeate (purified water) is discharged into waterways. It is essential for the permeate water to be pH balanced and cooled to avert thermal pollution of waterways and altering its pH.
Cleaning [edit]
Cleaning of the membrane is done regularly to prevent the accumulation of foulants and reverse the degrading effects of fouling on permeability and selectivity.
Regular backwashing is often conducted every 10 min for some processes to remove block layers formed on the membrane surface.[viii] By pressurising the permeate stream and forcing it dorsum through the membrane, accumulated particles can be dislodged, improving the flux of the procedure. Backwashing is limited in its ability to remove more circuitous forms of fouling such as biofouling, scaling or adsorption to pore walls.[25]
These types of foulants require chemical cleaning to exist removed. The common types of chemicals used for cleaning are:[25] [26]
-
- Acidic solutions for the control of inorganic scale deposits
- Alkali solutions for removal of organic compounds
- Biocides or disinfection such as chlorine or peroxide when bio-fouling is axiomatic
When designing a cleaning protocol it is essential to consider:
Cleaning time – Acceptable fourth dimension must exist allowed for chemicals to collaborate with foulants and permeate into the membrane pores. However, if the process is extended beyond its optimum duration it tin can lead to denaturation of the membrane and deposition of removed foulants.[25] The complete cleaning cycle including rinses between stages may take as long as 2 hours to complete.[27]
Aggressiveness of chemic treatment – With a high degree of fouling it may be necessary to employ aggressive cleaning solutions to remove fouling material. However, in some applications this may not be suitable if the membrane material is sensitive, leading to enhanced membrane ageing.
Disposal of cleaning effluent – The release of some chemicals into wastewater systems may be prohibited or regulated therefore this must be considered. For example, the employ of phosphoric acid may event in high levels of phosphates entering water ways and must be monitored and controlled to forbid eutrophication.
Summary of mutual types of fouling and their respective chemical treatments [8]
| Foulant | Reagent | Time and Temperature | Mode of Activeness |
|---|---|---|---|
| Fats and oils, proteins, polysaccharides, bacteria | 0.5 M NaOH with 200 ppm Cl2 | 30–sixty min 25–55 °C | Hydrolysis and oxidation |
| Dna, mineral salts | 0.one–0.five K acrid (acetic, citric, nitric) | 30–60 min 25–35 °C | Solubilization |
| Fats, oils, biopolymers, proteins | 0.one% SDS, 0.1% Triton Ten-100 | xxx min – overnight 25–55 °C | Wetting, emulsifying, suspending, dispersing |
| Jail cell fragments, fats, oils, proteins | Enzyme detergents | 30 min – overnight 30–twoscore °C | Catalytic breakdown |
| Deoxyribonucleic acid | 0.5% DNAase | thirty min – overnight 20–40 °C | Enzyme hydrolysis |
New developments [edit]
In order to increase the life-cycle of membrane filtration systems, energy efficient membranes are being adult in membrane bioreactor systems. Applied science has been introduced which allows the power required to aerate the membrane for cleaning to be reduced whilst withal maintaining a high flux level. Mechanical cleaning processes have also been adopted using granulates equally an alternative to conventional forms of cleaning; this reduces energy consumption and too reduces the expanse required for filtration tanks.[28]
Membrane properties accept also been enhanced to reduce fouling tendencies past modifying surface properties. This can exist noted in the biotechnology manufacture where membrane surfaces have been altered in order to reduce the amount of protein binding.[29] Ultrafiltration modules have too been improved to allow for more membrane for a given expanse without increasing its risk of fouling by designing more efficient module internals.
The electric current pre-treatment of seawater desulphonation uses ultrafiltration modules that have been designed to withstand high temperatures and pressures whilst occupying a smaller footprint. Each module vessel is self supported and resistant to corrosion and accommodates easy removal and replacement of the module without the cost of replacing the vessel itself.[28]
See as well [edit]
- List of wastewater handling technologies
References [edit]
- ^ Clever, K.; Jordt, F.; Knauf, R.; Räbiger, N.; Rüdebusch, G.; Hilker-Scheibel, R. (1 December 2000). "Process water production from river h2o by ultrafiltration and opposite osmosis". Desalination. 131 (1–3): 325–336. doi:10.1016/S0011-9164(00)90031-6.
- ^ Laîné, J.-M.; Vial, D.; Moulart, Pierre (1 December 2000). "Status after x years of operation — overview of UF technology today". Desalination. 131 (i–3): 17–25. doi:10.1016/S0011-9164(00)90002-Ten.
- ^ American Water Works Association Research Foundation ... Ed. group Joël Mallevialle (1996). Water handling membrane processes. New York [u.a.]: McGraw Hill. ISBN9780070015593.
- ^ Edwards, David; Donn, Alasdair; Meadowcroft, Charlotte (1 May 2001). "Membrane solution to a "significant risk" Cryptosporidium groundwater source". Desalination. 137 (i–3): 193–198. doi:10.1016/S0011-9164(01)00218-1.
- ^ Villecco F., Aquino R.P., Calabrò 5., Corrente M.I., D'Amore Chiliad., Grasso A., Naddeo V. (2020). "Fuzzy-assisted ultrafiltration of whey by-products recovery". Euro-Mediterranean Journal for Environmental Integration. 5. doi:10.1007/s41207-019-0138-five. S2CID 212655195.
{{cite periodical}}: CS1 maint: multiple names: authors list (link) - ^ a b c d e f Tamime, A. Y. (12 December 2012). Membrane Processing Dairy and Beverage Applications. Chicester: Wiley. ISBN978-1118457023.
- ^ a b Nigam, Mayank Omprakash; Bansal, Bipan; Chen, Xiao Dong (1 January 2008). "Fouling and cleaning of whey protein concentrate fouled ultrafiltration membranes". Desalination. 218 (1–three): 313–322. doi:10.1016/j.desal.2007.02.027.
- ^ a b c d east f 1000 h i j Cheryan, Munir (1998). Ultrafiltration and Microfiltration Handbook. CRC Press. ISBN1420069020.
- ^ Brian, P.L., 1965, Concentration polarization in reverse osmosis desalination with variable flux and incomplete salt rejection, Ind. Eng. Chem. Fund. 4: 439−445.
- ^ Rizvi, Anil Kumar; Pabby, Ana Maria; Sastre, Syed S.H., eds. (2007). Handbook of membrane separations : chemical, pharmaceutical, and biotechnological applications. Boca Raton, Fla.: CRC Press. ISBN978-0-8493-9549-9.
- ^ Bruijn, J P F; Salazar, F N; Borquez, R (September 2005). "Membrane blocking in ultrafiltration: A new approach to fouling". Food and Bioproducts Processing. 83 (3): 211–219. doi:10.1205/fbp.04012.
- ^ Antony, Alice; Depression, Jor How; Greyness, Stephen; Childress, Amy Eastward.; Le-Clech, Pierre; Leslie, Greg (1 Nov 2011). "Scale formation and control in loftier pressure membrane h2o treatment systems: A review". Journal of Membrane Science. 383 (1–ii): 1–16. doi:10.1016/j.memsci.2011.08.054.
- ^ Flemming, H.-C.; Schaule, One thousand.; Griebe, T.; Schmitt, J.; Tamachkiarowa, A. (ane Nov 1997). "Biofouling—the Achilles heel of membrane processes". Desalination. 113 (2–3): 215–225. doi:10.1016/S0011-9164(97)00132-X.
- ^ Baker, J.S.; Dudley, L.Y. (one September 1998). "Biofouling in membrane systems — A review". Desalination. 118 (ane–iii): 81–89. doi:10.1016/S0011-9164(98)00091-v.
- ^ Futselaar, Harry; Weijenberg, Dick C. (i September 1998). "Arrangement design for large-scale ultrafiltration applications". Desalination. 119 (ane–iii): 217–224. doi:10.1016/S0011-9164(98)00159-3.
- ^ a b c d Belfort, Georges (one February 1988). "Membrane modules: comparison of different configurations using fluid mechanics". Journal of Membrane Science. 35 (iii): 245–270. doi:ten.1016/S0376-7388(00)80299-9.
- ^ Koch Membrane Systems. "Membrane Products". Koch Membrane Systems. Retrieved ix Oct 2013.
- ^ US Department of the Interior Bureau of Reclamation. "H2o Handling Primer for Communities in Need" (PDF). Us Section of the Interior Agency of Reclamation. Retrieved 11 October 2013.
- ^ Con-Serv Manufacturing. "Operation and Maintenance Manual - UF-6-HF Ultrafiltration Organization" (PDF). Con-Serv Manufacturing. Retrieved ten October 2013.
- ^ Laîné; prepared by Joseph G. Jacangelo, Samer Adham, Jean-Michel (1997). Membrane filtration for microbial removal. Denver, CO: AWWA Research Foundation and American Water Works Association. ISBN0898678943.
{{cite volume}}: CS1 maint: multiple names: authors list (link) - ^ Water, Sydney. "Rosehill Recycled Water Scheme - Fairfield Recycled Water Plant" (PDF). Sydney Water.
- ^ a b c Nordin, Anna-Karin; Jönsson, Ann-Sofi (1 November 2006). "Example written report of an ultrafiltration establish treating bleach plant effluent from a pulp and newspaper mill". Desalination. 201 (i–3): 277–289. doi:10.1016/j.desal.2006.06.004.
- ^ Farahbakhsh, Khosrow; Adham, Samer S.; Smith, Daniel Due west. (June 2003). "Monitoring the Integrity of Depression-Pressure Membranes". Journal AWWA. 95 (6): 95–107. doi:10.1002/j.1551-8833.2003.tb10390.x.
- ^ American Water Works Association Research Foundation ... Ed. group Joël Mallevialle (1996). Water handling membrane processes. New York [u.a.]: McGraw Hill. ISBN0070015597.
- ^ a b c Cui, edited by Z.F.; Muralidhara, H.Southward. (2010). Membrane technology : a practical guide to membrane technology and applications in nutrient and bioprocessing (1st ed.). Amsterdam: Butterworth-Heinemann. pp. 213*254. ISBN978-ane-85617-632-3.
- ^ Gao, Wei; Liang, Heng; Ma, Jun; Han, Mei; Chen, Zhong-lin; Han, Zheng-shuang; Li, Gui-bai (1 May 2011). "Membrane fouling control in ultrafiltration engineering for drinking water product: A review". Desalination. 272 (1–3): 1–eight. doi:10.1016/j.desal.2011.01.051.
- ^ Wallberg, Ola; Jönsson, Ann-Sofi; Wickström, Peter (1 December 2001). "Membrane cleaning — a example study in a sulphite pulp mill bleach institute". Desalination. 141 (three): 259–268. doi:ten.1016/S0011-9164(01)85004-9.
- ^ a b Bennett, Anthony (1 November 2012). "Membrane applied science: Developments in ultrafiltration technologies". Filtration + Separation. 49 (6): 28–33. doi:ten.1016/S0015-1882(12)70287-2.
- ^ Ag, Due south (one September 2012). "Free energy-efficient membrane is designed for MBR systems". Membrane Technology. 2012 (9): 4. doi:10.1016/S0958-2118(12)70178-seven.
External links [edit]
-
 Media related to Ultrafiltration at Wikimedia Commons
Media related to Ultrafiltration at Wikimedia Commons
macdonaldforrind1950.blogspot.com
Source: https://en.wikipedia.org/wiki/Ultrafiltration

0 Response to "Easy 3 Credit Courses Uf Nature of Running"
Post a Comment